
Ketamine has achieved a major breakthrough in its advancement as a treatment for depression. Nasal spray esketamine (marketed as SPRAVATO™) was formally approved by the Federal Drug Administration (FDA) in March 2019 as a new therapy option for treatment-resistant depression (TRD). One of two molecules that comprise ketamine, esketamine has now been approved by the FDA to treat those who suffer from major depressive disorder (MDD) with acute suicidal ideation or behavior.
This is a win for anyone who suffers from suicidal ideation. Suicide is the 12th leading cause of death in the US for all ages. Every day, approximately 123 Americans die by suicide; that’s one death every 12 minutes. As the first new type of antidepressant approved in the last 30 years, esketamine opens up more options for those who suffer from depression and suicidal thoughts.
As a provider of Ketamine Infusion Therapy, we have seen the drug’s benefits in treating depression for many years. We are excited by this news for several reasons. Clinical studies and trials continue to prove that low-dose ketamine, or esketamine in the SPRAVATO™ formulation, offers fast-acting and highly effective relief from depressive symptoms. Press-worthy news and publicity also helps position ketamine as a top antidepressant contender instead of the inaccurate and exaggerated label of the party drug it’s commonly known as.
What Is the Difference Between Ketamine and Esketamine?
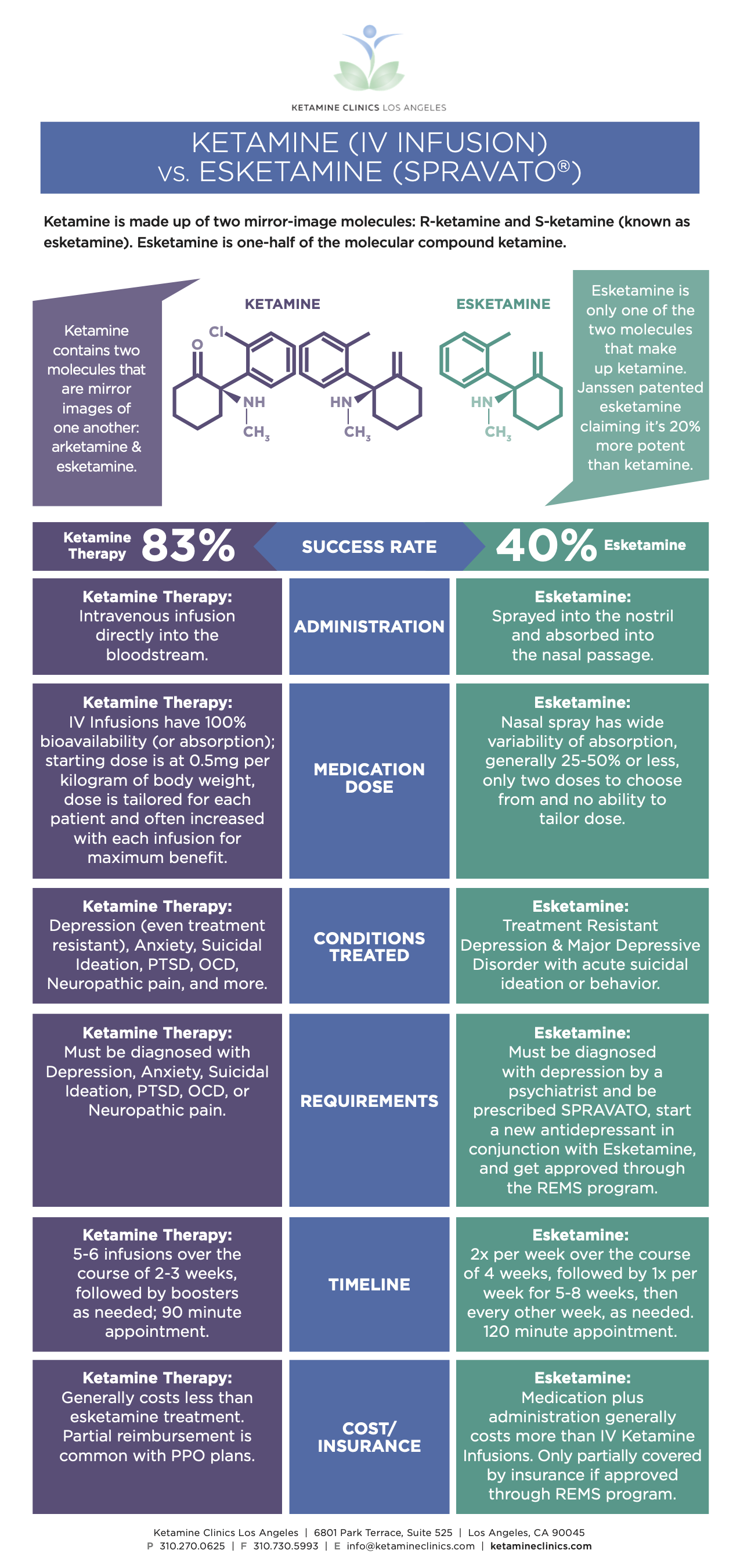
Ketamine is FDA-approved as an anesthetic. Its use for depression via the IV route of administration is off-label. One third of psychiatric medications are prescribed off-label in the US, so it's a very common practice. This simply means the medicine is found to be helpful for conditions or demographics it wasn't originally approved for. Esketamine is essentially half of the molecular composition of ketamine and is approved by the FDA to treat depression and suicidal ideation.
Ketamine is made up of two mirror-image molecules: R-ketamine and S-ketamine (known as esketamine). Essentially, esketamine (“S-ketamine”) is literally one-half of the molecular compound ketamine.
What Is SPRAVATO™?
SPRAVATOTM is a prescription medicine composed of esketamine to help treat adults who suffer from TRD and MDD with acute suicidal ideation or behavior. Its route of administration (ROA) is intranasal, self-administered as a nasal spray under the careful supervision of a clinician in a doctor’s office.
SPRAVATOTM is patented by Johnson & Johnson’s drug company, Janssen Pharmaceuticals Inc.
Why Isn’t Ketamine IV Infusion Therapy FDA-Approved?
In order to receive FDA approval, medicines need to undergo the approval process for each use and for each demographic. This process costs an average of $19 million dollars. Pharmaceutical companies aren’t willing to spend that money because ketamine isn’t covered by a patent and is widely used—meaning there’s no way Big Pharma can recoup their investment.
In essence, SPRAVATOTM is Big Pharma introducing an old drug, very slightly modified, via a new route of administration, so that they can obtain a patent.
Depression Treatment: Ketamine Infusion Therapy vs. Esketamine Nasal Spray
When comparing Ketamine Infusion Therapy and esketamine, both help treat depression by restoring the balance of neurotransmitters required for a healthy, well-functioning brain. To see the difference between their routes of administration, average success rates, known side effects, and other variables, check out our Depression Treatment Comparison Guide.
Though a nasal spray seems less invasive than an intravenous (IV) infusion, there are certain limitations and requirements that need to be met by both the patient and the clinic:
- To qualify for SPRAVATOTM, patients must currently be taking an antidepressant and be diagnosed as treatment-resistant or having MDD with suicidal ideation.
- Patients require a two-hour office visit where the doctor supervises the patient's administration of the nasal spray and monitors the patient. The minimum commitment for this treatment is two visits per week for 4 weeks, then once a week for 5-8 weeks, then every two weeks at 9 weeks until...? There is no clear end date for this treatment.
- Spravato is at best shown to be around 40% effective, which is about half of the efficacy of IV Ketamine Infusions in research papers and less than half of the results we see in clinic.
- All patients are limited to a fixed dose of either 56mg or 84mg, so the dose cannot be personalized.
- In order to continue managing known and potential side effects of SPRAVATO™, all patients seeking to start treatment will be enrolled in a Risk Evaluation and Mitigation Study (REMS) required by the FDA.
Because SPRAVATOTM is so new, the studies—while encouraging—are still limited. Early evidence shows both treatment modalities offer fast-acting and highly effective relief from depression and mood disorders. SPRAVATOTM and its required accompanying oral antidepressant complicate assessments, making it impossible to pinpoint whether it’s the esketamine or new oral medication producing the improvement.
There have been no direct tests comparing SPRAVATOTM with IV Ketamine. We can only compare FDA trials with our own experience and other studies involving ketamine as a treatment for depression. We do know that Intravenous Ketamine Infusion Therapy ensures 100% of the medication enters the blood and targets the brain, whereas SPRAVATOTM is likely to be less than 50% bioavailable due to its absorption through nasal membranes.
Although Ketamine Clinics Los Angeles is an approved SPRAVATOTM provider, and we do offer this treatment, patients have not chosen it over IV Infusions of Ketamine for the above and below reasons. Ketamine Infusion Therapy remains the gold standard with:
- An 83% success rate of relieving symptoms in patients in our clinic
- Fewer clinical visits (and shorter visits) needed to see results (typically six visits in a series)
- Overall lower costs
- No known long-term side effects
- Long-lasting relief between treatments (average of 3-4 months)
- No requirement to be on an antidepressant in order to receive treatment
- Personalized and precisely controlled dosing and treatment
It’s exciting to see ketamine continue to be recognized as a valuable, life-saving depression treatment. If you’re interested in learning more about the differences between SPRAVATOTM and Ketamine Infusion Therapy, contact our patient care staff today.


